
eSUNMed Biotechnology (Shenzhen)Co.,Ltd.
China Biomaterials Society Unit Memberô eSUNMed Biotechnology (Shenzhen)Co.,Ltd. (hereinafter referred to as “eSUNMed”), a subsidiary of Shenzhenô Esunô Industrialô Co.,ô Ltd., leverages Guanghua Weiye’s years of experience in the research and application of polylactic acid (PLA), polyglycolic acid (PGA), and polycaprolactone (PCL). The company focuses on the research and industrialization of biomedical monomers (such as lactide, glycolide, caprolactone), polymers (PLA, PCL, PGA, PLGA), 3D printing materials (PEEK, PLA, PCL, etc.), and downstream medical devices. Committed to the development and application of biomedical materials, the companyãs mission is to “develop biomedical materials and create a green, healthy life.” It aims to empower the medical industry with material science and contribute to human health, striving to become one of the leaders in the field of biomedical materials.
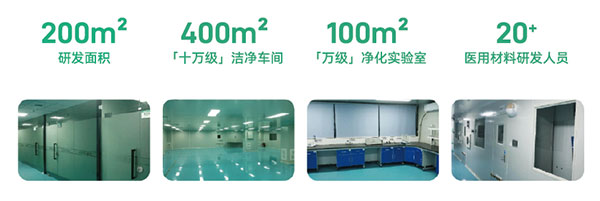
The companyãs main operating base covers an area of more than 2,000 square meters, with nearly 400 square meters of class 100,000 cleanrooms and a 100-square-meter class 10,000 purification laboratory. The facilities meet the production standards for pharmaceutical excipients and sterile medical devices. Additionally, the company is equipped with advanced equipment that ensures stable support for product research, production, and application.

eSUNMed‘s main products and services include four major series:
- Biomedical Monomers (such as lactide, glycolide, caprolactone, etc.)
- Biomedical Polymers (PLA, PCL, PGA, PLGA, etc.)
- Medical 3D Printing Consumables (PEEK, PLA, PCL, dental resins, etc.)
- Biomedical Material Processing (microspheres, injection molding, electrospinning, 3D printing, etc.).


eSUNMed‘s polycaprolactone (PCL) microspheres successfully passed the National Medical Products Administration (NMPA) medical device main document registration on July 16, 2024, with the registration number M2024210-000. With this,ô eSUNMed has completed the main document registration for its four major products: PLLA implant-grade polymers and microspheres, and PCL polymers and microspheres. The next step forô eSUNMed is to continue improving the registration and filing of other potential products, including PDLLA raw materials and microspheres, PEG copolymers and microspheres, and more.

Biomedical polymer materials possess excellent biocompatibility, causing no secondary harm to human health, while also improving the efficiency of clinical healthcare with greater humanistic care features. With the continuous development of materials science, more advancements and innovations will be brought to the medical industry!
Currently, the government has rolled out several policies to encourage the development of biomedical materials.ô eSUNMed will rely on the advantages of Shenzhen’s high-end medical device industry cluster and Guanghua Weiye’s years of technological accumulation in biomaterials. We will continue to introduce top-tier R&D talent and advanced equipment, ramp up our product development efforts, break through key technical barriers, and contribute to the development of the biomedical materials industry in China and to human health.
Should you have any needs, feel free to contact us. At the same time,ô eSUNMed sincerely invites domestic and international medical institutions, research units, and universities to cooperate with us in project research and clinical application experiments. Users interested in our products or services can contact us via the contact information displayed below.

