Congratulations to eSUNMED for the successful registration of their Poly-L-lactic acid (PLLA) material with the National Medical Products Administration’s main medical device document on May 11, 2024. The main document registration number is M2024152-000.
This is another registered product following the PCL’s main document filing. eSUNMED is dedicated to providing raw material services for the biomedical industry.
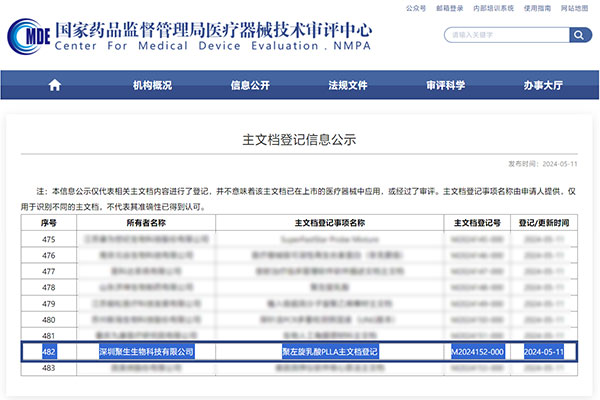


Information source: The official website of the Medical Device Technical Review Center of the National Medical Products Administration.
https://www.cmde.org.cn/xwdt/zwddjxxgs/20210413105900664.html
eSUNMED ‘s medical Poly-L-lactic acid (PLLA) material obtaining this qualification indicates that its raw materials meet the national quality standards, and the production process is standardized, comprehensive, traceable, and the technical documentation is complete. Furthermore, this also means that eSUNMED ‘s medical PLLA material can be used as a standard raw material for the research and development and production of downstream related products. With key raw materials that are risk-controllable, quality-reliable, and well-documented, it simplifies the product registration declaration information, reduces the difficulty of approval, and shortens the market cycle of new products.

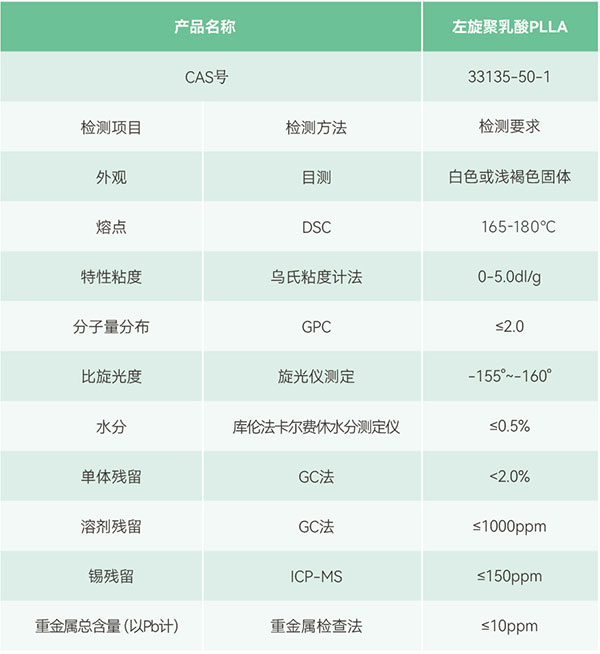
Introduction to eSUNMED ‘s biomedical PLLA material
Poly-L-lactic acid (PLLA) is produced by the ring-opening polymerization of L-lactide. The product has good biocompatibility and, once degraded in the body, its metabolites are excreted without any harm or toxic side effects. Therefore, it is widely used in the medical field for applications such as disposable infusion devices, non-removable surgical sutures; drug-controlled release and slow-release agents; tissue engineering scaffolds, cardiovascular stents, bone fixation and bone repair materials (such as absorbable screws, absorbable bone plates), injectable microcapsules, microspheres, implants, and elastomers for supporting animal organs.
eSUNMED PLLA material is characterized by being non-toxic, non-irritating, biodegradable and absorbable, with high strength, good plasticity, and easy to process and mold. Its degradation cycle is estimated to be 24-48 months, and by adding different modifiers, the product’s degradation cycle can be more flexibly controlled. PLLA degrades within the body to ultimately form carbon dioxide and water, demonstrating good biocompatibility.


Applications of PLLA Material in Medical Devices
1.Application of PLLA Microspheres in Medical Aesthetics
Poly-L-lactic acid (PLLA) microspheres are currently one of the most sought-after materials in the domestic medical aesthetics market. Once PLLA microspheres penetrate the deeper layers of the skin, they continuously induce the body’s fibroblasts to secrete and synthesize new collagen. This newly formed collagen gradually fills the volume of the previously missing areas and repairs the skin’s elasticity network. From the inside out and from the superficial to the deep layers, it fades facial wrinkles and depressions, restoring the skin to a youthful state and thus achieving an anti-aging effect.
Poly-L-lactic acid (PLLA) microspheres are regenerative materials that stimulate endogenous collagen regeneration. Collagen will naturally fill the volume deficits, providing a realistic and natural effect. Additionally, as the microspheres degrade within the body, the metabolic products and pathways are clear, preventing issues such as facial “overcorrection” and stiffness due to residual cross-linking agents.
2.Application Example of PLLA as Bone Fixation and Repair Material
Taking absorbable bone fixatives as an example: Absorbable internal fixatives based on PLLA material are biocompatible, non-irritating to tissues, and can be 100% absorbed by the human body. They degrade slowly within the body, with strength gradually decreasing, and eventually degrade into carbon dioxide and water. As the implant degrades, the reaction gradually shifts to the healing fracture surface, which is beneficial for increasing bone density, preventing osteoporosis, and overcoming the obstruction of local stress and re-fracture after bone healing.
Such products begin to be absorbed 3-6 months after being implanted in the human body, eliminating the need for a second removal, and are gradually replacing non-absorbable steel and titanium alloy products in medical clinical applications.

In March 2022, eSUN’s subsidiary, eSUNMed Biotechnology (Shenzhen)Co.,Ltd. (brand “eSUNMED”), was officially established. Currently, eSUNMED can provide biomedical monomers, biomedical polymers, and medical 3D printing materials. Additionally, it can offer medical processing services according to customer needs, such as medical 3D printing consumables processing, medical electrospinning processing, medical injection molding services, medical materials/microspheres processing, medical filament/tube/wire processing, and medical 3D printing services.
eSUNMED has a nearly 400 square meter class 100,000 standard clean workshop and a 100 square meter class 10,000 purification laboratory, meeting the standards for excipient production and sterile medical device production in GMP-compliant facilities, in line with the biomedical polymer materials industry standards. Additionally, it possesses various advanced equipment, providing stable support for the enterprise’s product research and development, production, and application.

