On March 19th, the medical-grade PLLA and PCL microspheres product standard review meeting was successfully held by eSUNMED. The review meeting was chaired by General Manager Chen Rui, with Dr. Kevin Yang serving as the head of the review team. Dr. Xie, the Technical Director, along with departments related to technology, quality, and production, collectively discussed and determined the technical specifications and related quality standards for the medical-grade PLLA and PCL microspheres produced by eSUNMED.

1.ô Referencing the standards “GB/T 29284-2012 Polylactic Acid” and “YY/T 0661-2017 Surgical Implants – Semi-crystalline Polypropylene Carbonate Polymers and Copolymer Resins,” the following technical specifications for medical-grade PLLA microspheres have been established. The related inspection items and specific testing methods are as follows:
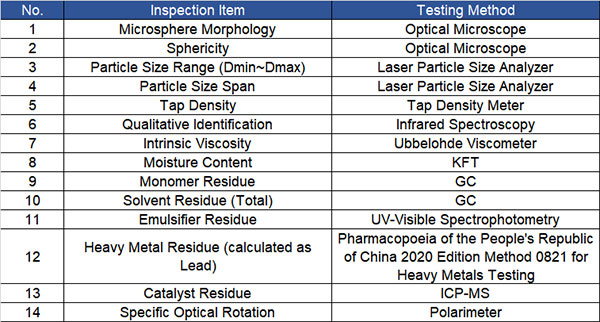
2.ô Based on “GB/T 37642-2019 Polycaprolactone” and “YY/T 0661-2017 Surgical Implants – Semi-crystalline Polycaprolactone Polymer and Copolymer Resins,” the technical specifications for medical-grade PCL microspheres have been determined. The related inspection items and specific testing methods are as follows:
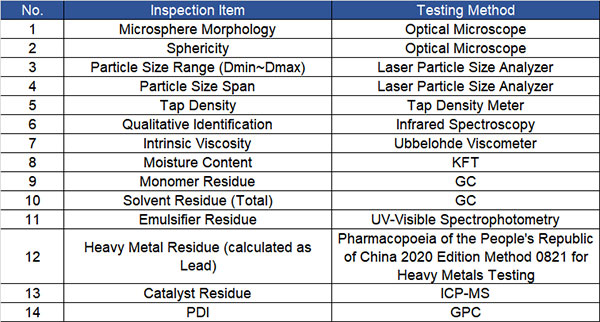
By establishing enterprise standards for medical-grade PLLA and PCL microsphere products, it helps to further standardize and improve the production process system, providing stable support and assurance to downstream applications with excellent product quality.
The biomedical polymer materials industry is developing rapidly. In the medical field, injection microspheres, embolic microspheres, IVD microspheres, and other products derived from underlying technologies have a wide range of application prospects in areas such as drug preparation, payload delivery, and analytical testing.
In the medical aesthetics industry, traditional injectable materials such as hyaluronic acid and collagen are gradually being replaced by safer and more superior regenerative injectable materials. As people’s enthusiasm for cosmetic medical consumption increases in the future, and the demand for anti-aging and related products grows, biodegradable medical injectable microspheres will have a broad development space.
Poly-L-lactic acid (PLLA) microspheres are currently one of the hottest materials in the domestic medical aesthetics market. Once PLLA microspheres penetrate the deeper layers of the skin, they continuously induce the body’s fibroblasts to secrete and synthesize new collagen. This newly formed collagen gradually fills the volume of the previously missing areas and repairs the skin’s elasticity network. From the inside out and from the superficial to the deep layers, it fades facial wrinkles and depressions, restoring the skin to a youthful state and thus achieving an anti-aging effect.
Poly-L-lactic acid (PLLA) microspheres are regenerative materials that stimulate endogenous collagen regeneration. The collagen naturally fills the volume deficits, providing a realistic and natural effect. Moreover, as the microspheres degrade within the body, the metabolic products and pathways are clear, avoiding issues such as facial “overcorrection” and stiffness that can occur due to residual cross-linking agents.
Polycaprolactone (PCL) microspheres have a wide range of applications in the medical field, including drug delivery devices, sutures or adhesion barriers, tissue engineering scaffolds, controlled drug release, and targeted delivery. In the field of medical aesthetics, products based on PCL can correct signs of facial aging, such as volume loss and contour relaxation, by stimulating the production of collagen, providing immediate and lasting natural effects.

eSUNMED has independently developed a variety of microsphere preparation methods, such as emulsion evaporation, membrane emulsification, and microfluidic control, which can produce droplets with controllable sizes and uniform particle diameters. Through research on droplet solidification processes, they have prepared blank microspheres of PLLA, PCL, PLGA, etc., with smooth surfaces, high sphericity, and a controllable D50 range of 10-100 ö¥m. By employing special particle size classification processes, the particle size distribution has been further narrowed, achieving a span value of less than 0.7.


In conclusion, eSUNMED welcomes research institutions or enterprises and institutions with needs to contact us for product customization or new application development.
At the same time, eSUNMED will continue to strictly implement various standard regulations, continuously improve product testing capabilities and product quality, strengthen the construction of the standard system, and provide users with high-quality and stable products and services!

