On February 6, 2024, eSUNMED successfully registered its self-developed medical-grade polycaprolactone (PCL) material with the National Medical Products Administration’s primary documentation for medical devices, under the registration number M2024050-000.

The eSUNMED’s medical-grade polycaprolactone (PCL) material has obtained the qualification, indicating that its raw materials meet the national quality standards, and the production process is standardized, comprehensive, traceable, and well-documented. Additionally, this signifies that eSUNMED’s medical PCL material can serve as a standard raw material for the research and production of downstream related products. As a key raw material that is risk-controllable, reliable in quality, and complete in documentation, it simplifies the product registration declaration information, reduces the difficulty of approval, and shortens the market cycle for new products.
01¬†Introduction to eSUNMED’s Medical PCL Material
eSUNMED’s medical-grade polycaprolactone (PCL) material, developed and produced by the company, possesses excellent biocompatibility, shape memory, and biodegradability. The PCL material is soft, easy to process, performs excellently, has strong crystallinity, and degrades slowly. Its degradation in the body occurs in two stages: the first stage is characterized by a continuous decrease in molecular weight without deformation and weight loss; the second stage begins when the molecular weight decreases to a certain level, the material starts to lose weight, and is gradually absorbed and excreted by the body, making it an ideal biomaterial for medical use.

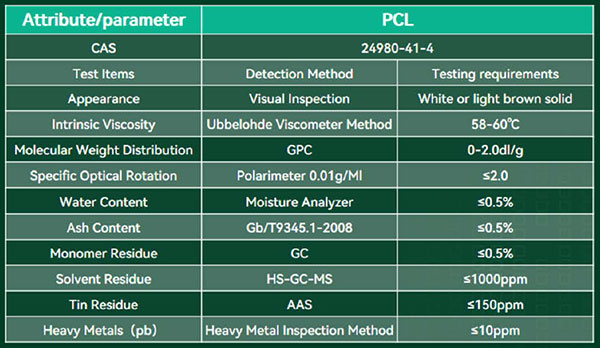
eSUNMED has adopted a continuous polymerization process to replace the intermittent batch polymerization, resolving the issue of uneven material mass and heat transfer. This ensures stable production batches, guarantees the stability of batch materials, and achieves a product molecular weight distribution of less than 2.0.
 
02 The applications of PCL material in medical devices
1. Medical Sutures
eSUNMED’s medical-grade polycaprolactone (PCL) exhibits excellent biocompatibility and biodegradability, making it suitable for use in medical devices such as sutures. For instance, medical sutures can be stretched to 200% before setting. After surgery, as the body temperature increases, the shape memory of the sutures activates, gradually tightening and closing the wound.
Advantages:
вС† Sensitive to temperature with good shape memory function.
вС° Good biocompatibility and biodegradable properties.

2.3D Printed Bone Repair Scaffolds
PCL’s controllable degradation rate, high flexibility, adjustable elasticity, and tensile strength make it ideal for bone tissue engineering scaffolds.

3.Drug Carriers
The delayed and controlled release of drugs is extremely important for cancer treatment. By managing the release of drugs, it allows the medication to be released over a long period in a specific part of the body, maintaining a consistent concentration in the blood, thereby reducing the frequency of dosing and avoiding uneven intake. Encapsulating drug molecules within PCL microspheres can achieve slow and sustained drug release, enhancing the therapeutic effect and reducing side effects. PCL possesses good mechanical strength, elasticity, and flexibility, and can also encapsulate drugs within nanofiber drug carriers through electrospinning, creating dressings with antibacterial activity and lasting effects.
4.Cosmetic Injectable Fillers
Polycaprolactone microsphere soft tissue fillers represent a new generation of collagen biostimulant products, offering significant and lasting effects on facial volume loss, wrinkles, and contouring caused by aging. The PCL microspheres are uniformly suspended in a CMC gel carrier, providing immediate filling effects upon injection. As the CMC gel is degraded and absorbed, the PCL microspheres continue to stimulate fibroblasts, promoting the regeneration of endogenous collagen and forming a collagen scaffold to fill depressions and improve skin texture.
Through research on cutting-edge technologies such as membrane emulsification and microfluidics for the preparation of microsphere emulsion droplets,¬†eSUNMED can produce droplets with controllable size and uniform particle size. Through the research of droplet solidification process, PCL blank microspheres with smooth surface, high roundness, and controllable D50 in the range of 10-100 ќЉm are prepared. The particle size distribution is further narrowed through special particle size grading process, with a span value that can reach within 0.7. It is widely used in medical beauty products such as regenerative injections.

Polycaprolactone PCL microspheres electron microscope photo
 
 
03 About eSUNMed
In March 2022, a subsidiary of eSUN, eSUNMed Biotechnology (Shenzhen)Co.,Ltd. (eSUNMed), was officially established. Currently, eSUNMed provides monomer raw materials, biodegradable polymers, 3D printing biomaterials, and can also offer biomedical 3D printing services according to customer needs, including medical 3D printing filaments processing, medical electrospin services, medical molding services, medical material/microsphere processing, medical wire/tube/filament processing, and medical 3D printing services.
For any inquiries, please feel free to contact us. Additionally, eSUNMed invites medical institutions, research institutions, colleges, and universities worldwide to collaborate on related projects and clinical application experiments. Interested users can also contact us through the provided contact information below.

