In July 2022, eSUNMed’s medical 3D printing filaments production line was successfully launched. Up to now, eSUNMed has established stable production capacity for medical 3D printing filaments, providing users with medical PEEK 3D printing filaments and medical PLA 3D printing filaments. Additionally, eSUNMed offers customized 3D printing services for medical implants based on user requirements.
This article will use eSUNMed’s medical PEEK 3D printing filaments as an example to briefly introduce the production process and related information of eSUNMed’s medical 3D printing filaments.
1. Introduction to Medical PEEK Raw Materials
To ensure product quality from the source, eSUNMed adheres to high standards and strict requirements when selecting raw materials. For instance, in the case of medical PEEK 3D printing filaments, the raw materials are sourced from brands with ISO10993 certification qualifications from both domestic and international markets. In terms of biocompatibility testing, these materials undergo testing by authoritative testing organizations with certifications such as CMA, CNAS, ANAB, FDA GLP, and OECD GLP, meeting global requirements for biocompatibility evaluations.

2. Standardized Production of Medical PEEK 3D Printing Filaments
eSUNMed has independently developed a medical high-temperature 3D printing filaments production line. All medical 3D printing filaments are produced in clean workshops that comply with GMP standards. The production process strictly follows the production standards for Class III medicaldevices.
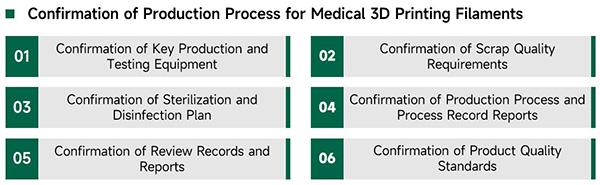
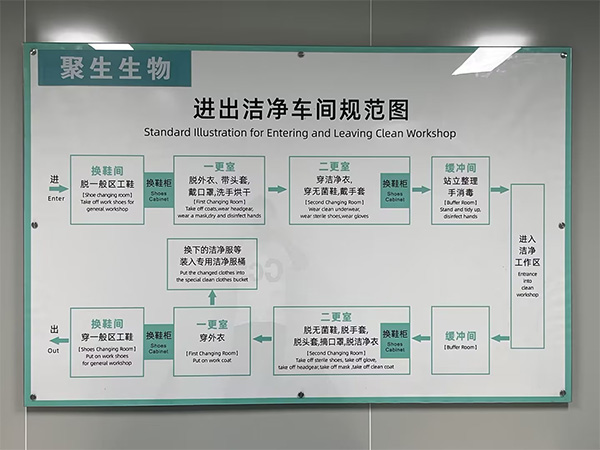


From production to packaging, strict quality control of products and materials, verification and confirmation of key processes, personnel and workshop management, eSUNMed maintains rigorous quality management to ensure strict control of process quality throughout the entire production process, ensuring product quality and efficient production.
3. Processing and Applications of Medical PEEK 3D Printing Filaments
As a new high-strength engineering plastic, Polyether Ether Ketone (PEEK) has excellent properties, including high-temperature resistance, self-lubrication, and chemical stability. PEEK materials also offer outstanding biocompatibility, and when compared to metal materials, PEEK materials have a modulus of elasticity that closely matches human bones. This mechanical performance meets the normal requirements of the human body, making it an ideal material for orthopedic implants. It can be used for manufacturing medical implants such as support structures, link plates, sternal fixation belts, and intervertebral fusion devices.
In the application of PEEK in cranial repair, its advantages compared to traditional titanium materials include:
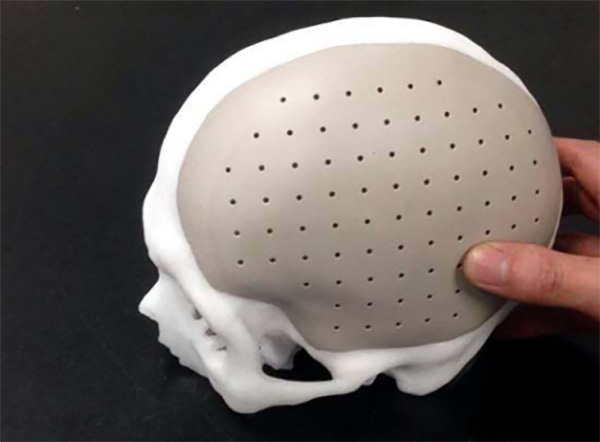
1.Biocompatibility: PEEK materials have good biocompatibility, reducing the body’s rejection response to implanted materials and lowering the risk of postoperative complications.
2.Modulus of Elasticity: PEEK’s modulus of elasticity is close to that of human bones, enabling better biomechanical adaptation to surrounding bone and reducing potential stress-related problems.
3.Lightweight: PEEK materials are relatively lightweight, reducing the burden on the implant site and aiding patient recovery.
4.Formability: PEEK materials have excellent formability, enabling customized design to suit individual patient needs and adapt to different cranial defect shapes.
5.X-ray Transparency: PEEK material exhibits excellent transparency to X-rays, allowing doctors to perform post-operative imaging checks to ensure the success of the surgery.
6.Corrosion Resistance: PEEK materials have strongly resist chemical corrosion, reducing postoperative degradation and damage to materials.
Traditional processing methods for PEEK materials include mechanical cutting, which has drawbacks such as thin bone plates, large shape radii, high costs, and material waste. Due to its high precision and freedom, additive manufacturing technology allows for both macro and microstructural customization of medical implants, ensuring better biomechanical compatibility with the patient’s defect site and meeting clinical personalized treatment needs. 3D printing technology to customize models for PEEK-related applications can achieve rapid manufacturing, complex structural design, cost savings, increased efficiency, reduced production time, and material consumption.

Currently, eSUNMed ‘s medical-grade PEEK 3D printer is also under testing.
The professional and mature development of eSUNMed’s medical 3D printing filaments production line will further promote the application of additive manufacturing technology in the medical field. A diverse range of medical 3D printing materials will provide more possibilities for personalized medicine. In the future, eSUNMed will continue to develop more medical 3D printing materials with excellent performance, such as PCL, PLGA, TPU, and more. Stay tuned!
About Shenzhen Jusing Biotechnology Co., Ltd.
In March 2022, a subsidiary of eSUN, Shenzhen Jusing Biotechnology Co., Ltd.(eSUNMed), was officially established. Currently, eSUNMed provides monomer raw materials, biodegradable polymers, 3D printing biomaterials, and can also offer biomedical 3D printing services according to customer needs, including medical 3D printing filaments processing, medical electrospin services, medical molding services, medical material/microsphere processing, medical wire/tube/filament processing, and medical 3D printing services.

For any inquiries, please feel free to contact us. Additionally, eSUNMed invites medical institutions, research institutions, colleges, and universities worldwide to collaborate on related projects and clinical application experiments. Interested users can also contact us through the provided contact information below.

